Revolutionizing Green Hydrogen Production
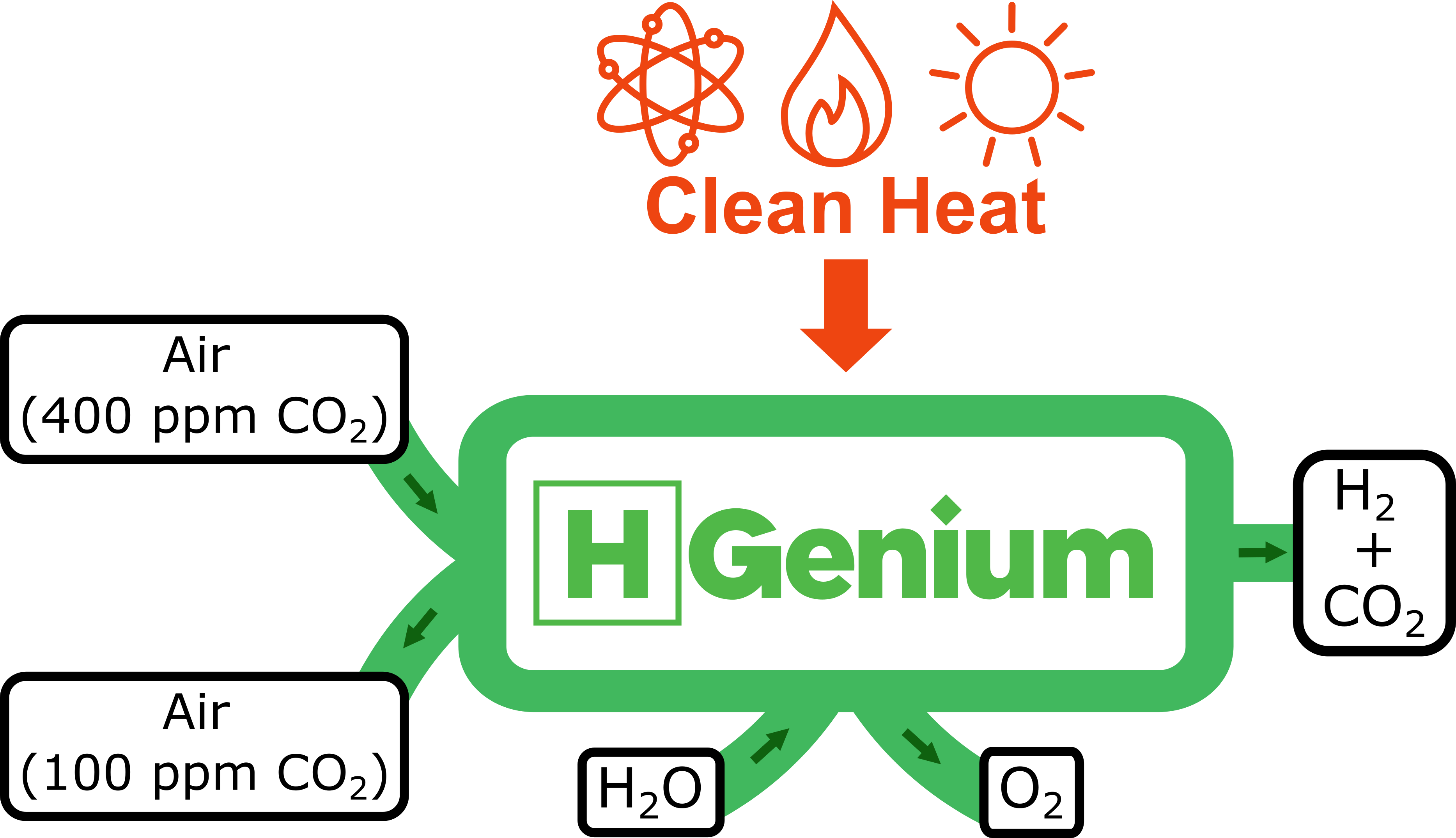
HGenium’s technology overcomes current challenges of thermochemical water splitting while pulling CO2 out of the air
HGenium’s process operates at a moderate temperature of ~850°C and requires no toxic or corrosive compounds. It relies on inexpensive materials; and can be executed with commercially mature equipment.
The net reaction of the cycle is the stoichiometric splitting of H2O to H2 and ½ O2 with no byproducts via shuttling alkali ions in and out of manganese oxides within a manganese reduction and oxidation cycle.
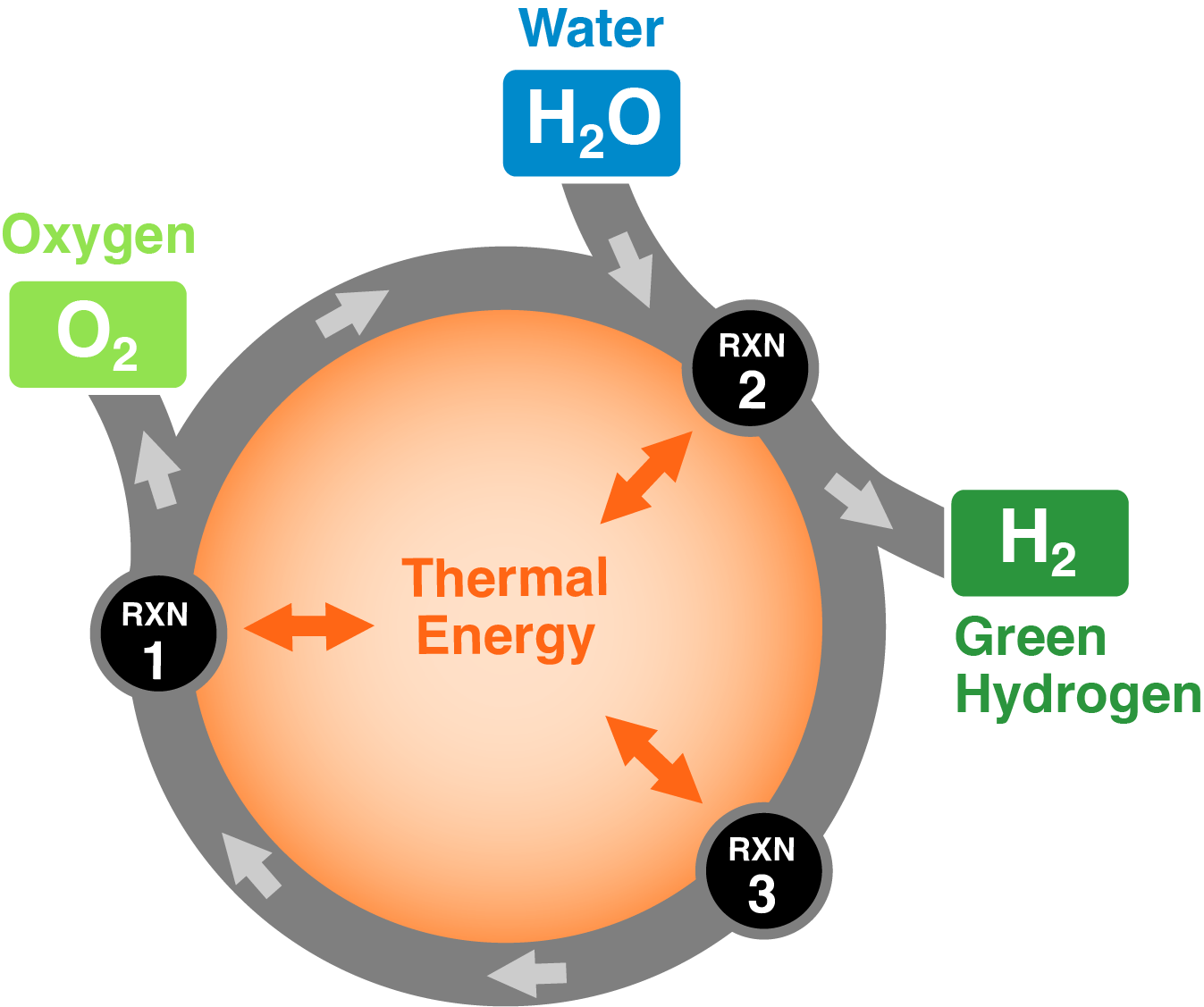
How thermochemical water splitting works
Using heat as the energy source, thermochemical water splitting uses a series of chemical reactions in a complete cycle to split water into hydrogen and oxygen. As the temperature required for any given cycle decreases the variety of applicable heat sources increases.
Challenges associated with existing technologies
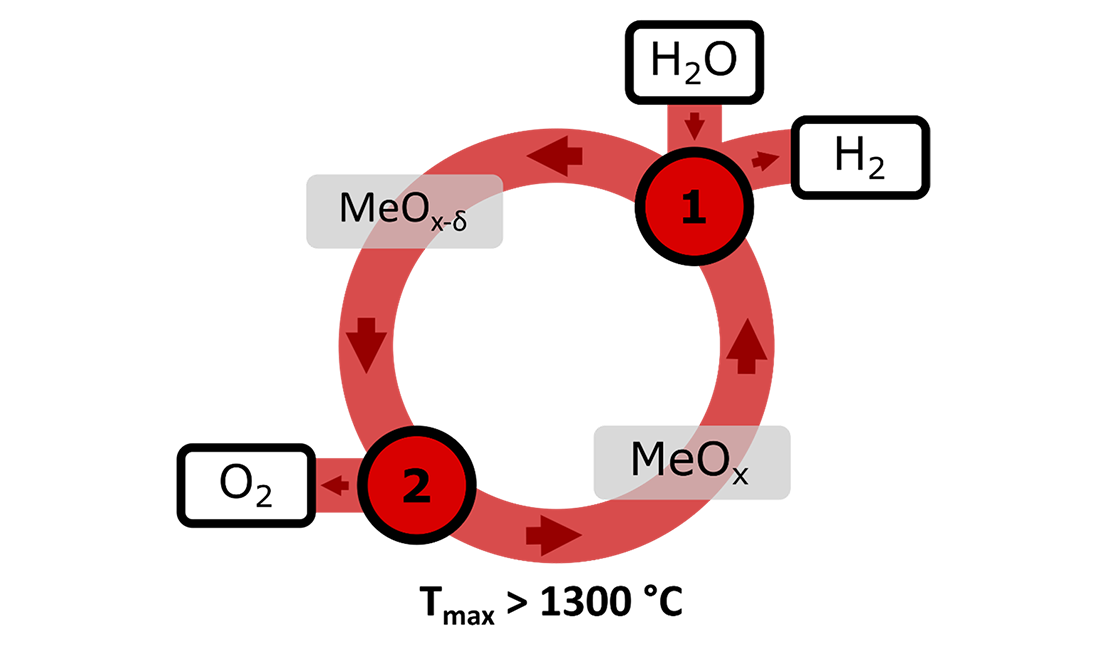
Extreme temperature metal oxide cycles
The extreme temperatures required to achieve water splitting in two-step metal oxide water splitting cycles necessitate the use of exotic reactor materials, and limit the number of applicable heat sources; creating a significant challenge for commercialization.
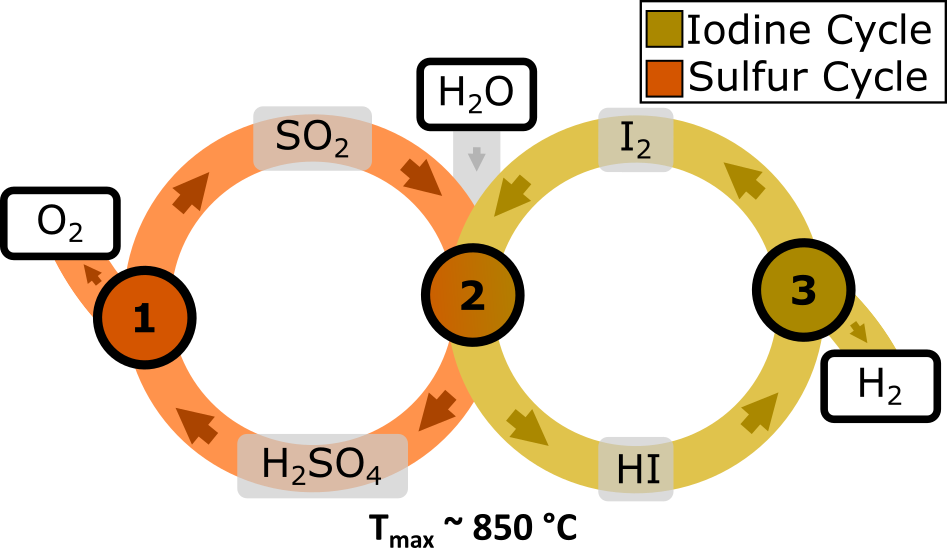
Intermediate temperature mineral acid cycles
Intermediate temperature (~850°C) mineral acid based thermochemical water splitting cycles use highly corrosive and toxic components to achieve water splitting imposing a barrier to real-world implementation of the technology.

